The Expansion Phase of Phase I Oncology Trials
The primary focus of any Phase I oncology trial is to find the Recommended Phase II Dose (RP2D), by ascertaining the maximum tolerated dose (MTD), the maximal dose with the dose limiting toxicities (DLT) not exceeding a pre-set limit. However, before proceeding to Phase II we would want to confirm that the RP2D is appropriate, there is a suitable population to use in the Phase II study, that the dose is efficacious and if there could be lower, less toxic doses with good efficacy [1] – this is where the Phase I Expansion Study comes in. Other areas of interest could include pharmacokinetics, pharmacodynamics, toxicities and other safety endpoints. Once a dose (or multiple doses) of interest have been found, additional cohorts potentially with a large sample size and/or stratified by prognostic factors to help determine Phase 2 populations can be added [2].
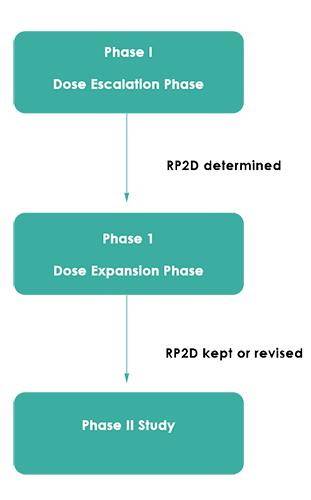
Figure 1 below outlines the basic structure of determining RP2D with a dose expansion phase. However, the Dose Expansion Phase structure can get quite complicated, and almost be as complex as the initial Dose Escalation Phase.
Figure 1: Simple Schematic of Phase 1 Study with Expansion Phase
 From a regulatory perspective, the FDA has not offered any binding recommendations for Phase I oncology trials to have an expansion, acknowledging for general trials the benefit of determining efficacy and a better understanding of the PK/Safety profiles but with the caveat of potentially over-exposing patients and potentially making poor inference [3]. There is also conflicting opinion within the medical community, with speedier drug development as a commonly cited benefit, with the potential risk of not providing scientifically justifiable information, especially as commonly no expansion sample size justification is provided [4,5] or the actual analysis itself is not planned [6]. However, there is also the benefit of reduced costs overall and allowing for better allocation of money to better compounds [5]. With a continued uptake of dose expansion phases, it is important to justify sample sizes and objectives; for example, only 12% of Phase I Oncology trials in 2006 had a dose expansion phase, as compared to 38% in 2011 [7].
From a regulatory perspective, the FDA has not offered any binding recommendations for Phase I oncology trials to have an expansion, acknowledging for general trials the benefit of determining efficacy and a better understanding of the PK/Safety profiles but with the caveat of potentially over-exposing patients and potentially making poor inference [3]. There is also conflicting opinion within the medical community, with speedier drug development as a commonly cited benefit, with the potential risk of not providing scientifically justifiable information, especially as commonly no expansion sample size justification is provided [4,5] or the actual analysis itself is not planned [6]. However, there is also the benefit of reduced costs overall and allowing for better allocation of money to better compounds [5]. With a continued uptake of dose expansion phases, it is important to justify sample sizes and objectives; for example, only 12% of Phase I Oncology trials in 2006 had a dose expansion phase, as compared to 38% in 2011 [7].
In terms of validity of results from expansion phases, it has been argued that the sample sizes do not need to be large when considering efficacy [1,8], of which the vast majority of Phase I trials with an expansion phase considered in 2011 [9]. However, this is not to dismiss the concerns of statistical validity – as an example, if we were to have several cohorts with a small sample size within each and Overall Survival, is it guaranteed that there will be enough events in each cohort to obtain statistically robust results? For more straightforward expansion studies where we just have one cohort, usually dosed at the RP2D found in the dose escalation study, this may not be too much of a concern. Alongside these concerns, we also must consider how the population of the expansion phase is defined; for example, if we exclude those with major protocol deviations, if subjects have specific forms of cancers etc; the latter being a common additional criteria for subjects to proceed into the dose expansion phase [6]. This could easily be decided on an ad-hoc basis; for example, if patients with colon cancer appear to respond better than other cancer types during the dose escalation phase. With small sample sizes, we could easily observe what appears to be efficacy by chance and hence this type of selection should be avoided where possible.
In 13% of the trials where the purpose of the expansion phase was to look at safety, the RP2D dose was modified based on the findings of the expansion phase, with 54% of trials reporting new toxicities [10]; simulations have also demonstrated that the MTD in 50% of trials was not found in the dose escalation stage alone [11]. Additionally, a meta-analysis of 381 cancer drugs showed that overall, having a dose expansion phase helped increase the chance of successful Phase II study [8]. Whilst this gives us a better impression of the safety profile and potentially prevents wastage in Phase II studies with inferior doses, We also have to consider how most Phase I trials are designed, with the majority using the 3+3 design – as discussed in the Quanticate whitepaper The Design of Phase I Oncology Studies, the 3+3 design has its own deficiencies in identifying the RP2D; if the model-based designs are better at identifying the RP2D than the 3+3 method then is an Expansion Phase strictly necessary (or at least, a large scale expansion phase)? Whilst having a clearer image of the safety profile is useful, over-exposing patients to medication and continuing the trial to find no change to the RP2D could be considered wasteful, especially considering that dose expansion phases could be as complex as the dose escalation section.
To conclude, having a dose expansion phase appears to provide benefit further down the line, providing further information about efficacy, safety, toxicity and pharmacokinetics prior to jumping into a potentially wasteful Phase II study. Most of the criticism appears to come from poor consideration of the statistical validity of the results and design of the dose expansion phase. However, even with the best design of the expansion phase, we still must consider the design of the dose escalation phase, with respect to assignment of doses and eventual assignment of RP2D – having a dose expansion phase to essentially correct for inadequate dose escalation design is something to be avoided. Avoiding these potential pitfalls is something Biostatistical Consulting can address to prevent it happening on your study!
If you feel a dose expansion phase may be suitable to help with a Phase I oncology study that you are designing, the Quanticate Statistics Department would be delighted to discuss your requirements and provide advice about possible ways to increase the chance of a successful study. For more information please submit an RFI.
References
[1] - Roychoudhury, S., & Lahiri, S. (Eds.). (2018). Statistical Approaches in Oncology Clinical Development: Current Paradigm and Methodological Advancement. CRC Press.
[2] – Boonstra P.S et al (2017). Statistical controversies in clinical research: building the bridge to phase II—efficacy estimation in dose-expansion cohorts. Annals of Oncology
[3] - Expansion Cohorts: Use in First-In-Human Clinical Trials to Expedite Development of Oncology Drugs and Biologics Guidance for Industry (2018) https://www.fda.gov/media/115172/download [Accessed 16AUG2019]
[4] - Percy Ivy, S. et al (2010) Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: a report from the Clinical Trial Design Task Force of the National Cancer Institute Investigational Drug Steering Committee. Clinical Cancer Research.
[5] - Iasonos, A., & O'Quigley, J. (2015). Clinical trials: Early phase clinical trials-are dose expansion cohorts needed?. Nature reviews Clinical oncology.
[6] O’Quigley, J. & Iasonos A. (2014). Dose expansion cohorts in Phase I trials. Memorial Sloan-Kettering Cancer Center, Dept. of Epidemiology & Biostatistics Working Paper Series
[7] Manji A et al (2013). Evolution of Clinical Trial Design in Early Drug Development: Systematic Review of Expansion Cohort Use in Single-Agent Phase I Cancer Trials. Journal of Clinical Oncology.
[8] - Bugano, D. et al. (2017). Use of expansion cohorts in phase 1 trials and probability of success in phase 2 for 381 anticancer drugs. Clinical Cancer Research.
[9] – Dahlberg, S.E. et al (2014). Evaluation of Statistical Designs in Phase I Expansion Cohorts: The Dana-Farber/Harvard Cancer Center Experience. Journal of the National Cancer Institute.
[10] – Wages,N et al (2018). Design considerations for early-phase clinical trials of immune-oncology agents. Journal for ImmunoTherapy of Cancer.
[11] – Hansen A., & Razak A (2014). The benefits of including expansion cohorts in Phase I oncology clinical trial design. Clinical Investigation.
Subscribe to the Blog
CONTACT DETAILS
Address - UK HQ:
9-11 Bancroft Court
Hitchin, Hertfordshire
SG5 1LH
United Kingdom
Tel: +44 (0)1462 440 084
Fax: +44 (0)1462 440 086
Email: enquiries@quanticate.com
Address - US HQ:
8601 Six Forks Rd
Suite 400
Raleigh, NC, 27615
United States
Tel: +1-919-882-2016
Email: info@quanticate.com
QUANTICATE
BLOG TOPICS
© 2024 Quanticate