IQ - Request A Walkthrough
Collaboration on clinical documents and sharing information has never been so easy with the IQ Portal
What you will learn in this walkthrough?
Request a walkthrough by a member of our team who will present the features and functionality of the IQ portal. Demonstrations are roughly 15 minutes for any drug development company interested. You will see how the IQ portal can improve the efficiency of your clinical trial at no additional costs with its core features of:
- Real-time access to data with dashboards and visualizations and knowledge needed to enable faster decisions and optimal commercialization
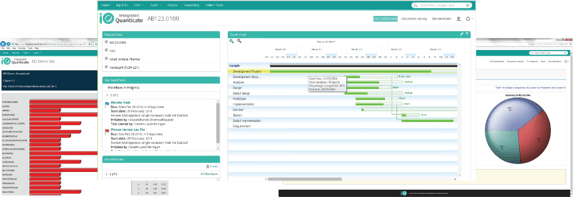
- A cloud portal that provides trial progress, quality and cost reports, detailed graphical representation can then be drilled down and filtered when the user requires to enable fast decisions
- Document development and review within a version-controlled environment provides a lean approach to your clinical trial process
- Workflows model business and regulatory processes, delivering controlled approvals and reviews with timely reminders
- Tables, Listings and Figures (TLFs) at no additional cost, review the progress of their study in advance of database close
If you are interested in a walkthrough of IQ – Integrated Quanticate®, please complete the form to the right.
IQ Walkthrough
CONTACT DETAILS
Address - UK (HQ):
Quanticate
Bevan House,
9-11 Bancroft Court
Hitchin, Hertfordshire
SG5 1LH
United Kingdom
Tel: +44 (0)1462 440 084
Fax: +44 (0)1462 440 086
Email: enquiries@quanticate.com
9-11 Bancroft Court
Hitchin, Hertfordshire
SG5 1LH
United Kingdom
Tel: +44 (0)1462 440 084
Fax: +44 (0)1462 440 086
Email: enquiries@quanticate.com
Address - US:
Quanticate
Forum I
8601 Six Forks Rd
Suite 400
Raleigh, NC, 27615
United States
Tel: +1-919-882-2016
Email: info@quanticate.com
8601 Six Forks Rd
Suite 400
Raleigh, NC, 27615
United States
Tel: +1-919-882-2016
Email: info@quanticate.com
QUANTICATE
BLOG TOPICS
© 2024 Quanticate
