This infographic explores Quality Process Improvement and Standard Operating Procedure (SOP) updates from a Clinical Research Organization (CRO) perspective. To confirm compliance to regulatory requirements, processes must be put into place - but how do we ensure our SOPs comply?
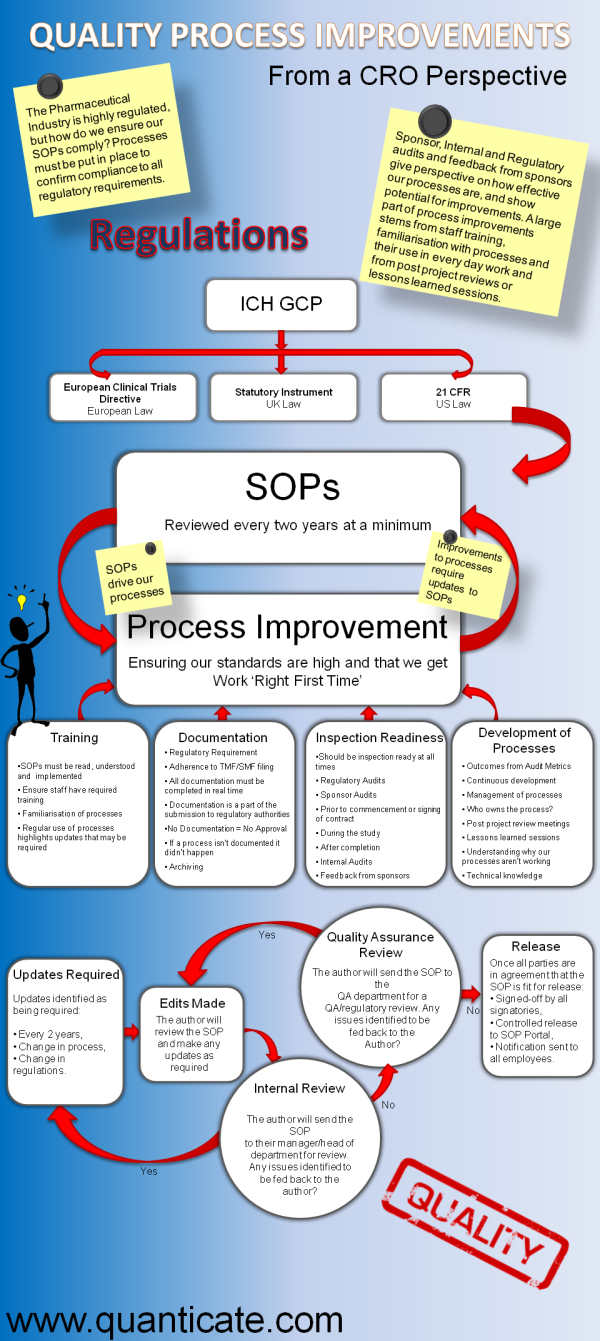
The pharmaceutical industry is very highly regulated, and in the last few years, the regulatory bodies have released many new or updated standards and laws which drives Standard Operating Procedure (SOP) changes to ensure company polices are in line with new legislation. These SOPs govern the internal processes of a CRO and at the ‘process improvement’ stage any changes made influenced by the external regulatory bodies circles back to SOP updates being required.
Training, documentation, inspection readiness activities, and development processes all help drive process improvement and any changes based on these internal factors within the CRO will drive SOP updates.
When a change to an SOP is required, the author will review and make the necessary updates. If any new legislation is brought into practice this would also result in a requirement for new SOPs to be written. These updated/new SOPs are then reviewed internally by the author’s manager or head of department, if there are comments from the review, further updates will be made and if the new version is approved, it is passed on to the Quality Assurance team for further review, to ensure that any regulatory requirements are adhered to correctly. At this stage the SOP could either be sent back to the author if edits are required or sent on to top management for final approval. Once all three approvals have been achieved, the SOP can be released for use by the CRO after sign-off by all signatories. The updated SOP is then placed in the SOP portal and all employees are notified of their requirement to read the new SOP.


Bring your drugs to market with fast and reliable access to experts from one of the world’s largest global biometric Clinical Research Organizations.
© 2025 Quanticate