Tables, listings and figures are part of day to day clinical submissions. Hence it would be useful if statisticians/clients could easily analyse the study data through different time points. This allows for better decisions because you are able to view outputs while the study is on going, this visualisation during a study can allow for great efficiencies as decisions can be made earlier in the clinical trial.Business intelligence produces analysis and provides credible information in making effective and high quality business (clinical) decisions. Typical BI infrastructure components are computer based techniques for gathering, cleansing, integrating, analyzing, reporting and sharing data. It provides the feasibility to create complex and interlinked graphics with the help of information maps; which will surface over the existing original data (via SDTM domains such as DM, AE, LB, VS) - without the need for modification of original data. The concept here lies in interlinking domains with the use of primary keys and surrogate keys which enable the statisticians and medical writers to easily create complex reports such as: patient profiles, interrelation between AE and effect on PK parameters, change in lab result with respect to exposure of the drug and patient pathway through different clinical studies.SAS Web Report Studio (WRS) is an SAS BI tool which empowers users with easy access to reports. It has the ability to answer questions in an evolving business (clinical research) environment, with the enhancement of multiple report linking and interactive report generation. It also provides the user the power of data synchronization and metadata security. Multiple users can access and distribute the reports in varied formats to make better decisions. This is because of the prompt point and click interface available in SAS WRS which reduces the buffer time between the programmer and statistician. Since the statistician does not have to wait for the programmer to re-do the updated changes required by the statistician - he can work on the possibilities himself.SAS BI can be the ultimate all in one tool to provide insight and make better decisions in clinical trials. It is the one tool which provides features like:
Related Blog Posts:
- Report linking
- Minimal coding (dropdown menu & options menu)
- Interactive reports capability (change the axis or the type of graph)
- Data integration (synchronization of updated data into respective domains)
- Data security (SAS Metadata level security)
It is also less time consuming. However, making legitimate and important decisions using a single application will always come with a price which is the only drawback for its complete implementation in a CRO Industry.
The interactive data exploration process
The infographic shows the process of how the data arrives into the Web Report Studio:
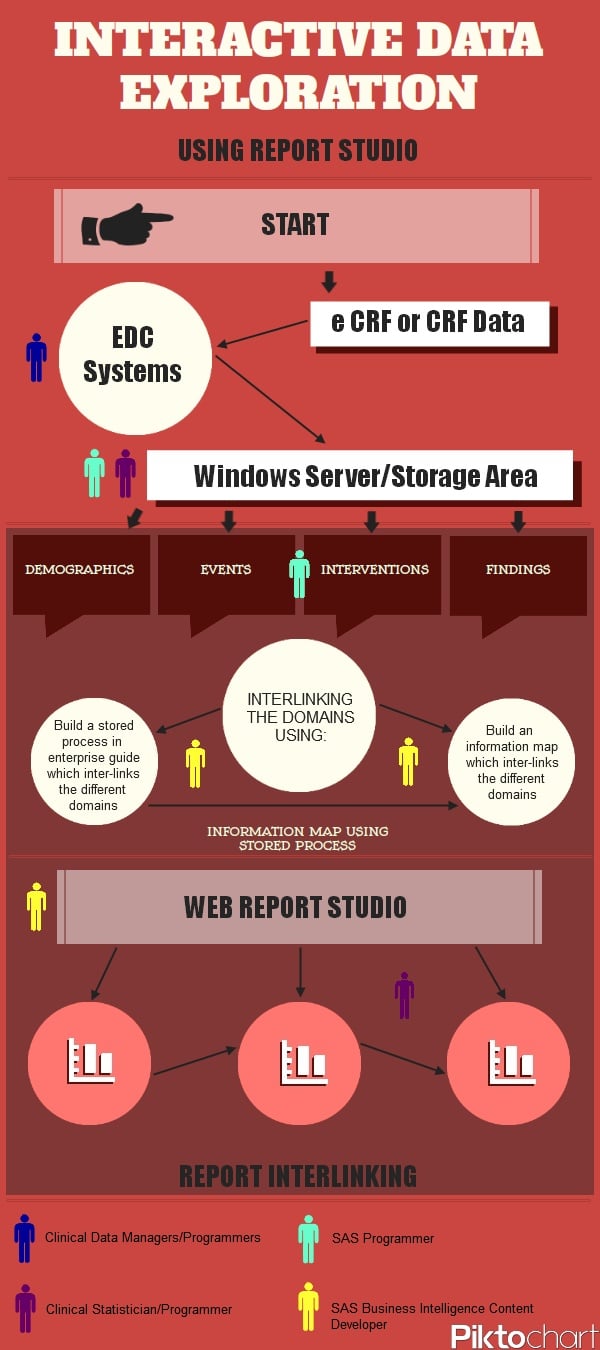
The coloured figures shown throughout the infographic, and also in the key at the bottom, represent people with the stated job title; and where they would be involved in the interactive data exploration process.
- CRF and eCRF data is collected into various electronic data capture systems by the clinical data managers.
- The data is then processed by either SAS programmers or statisticians/programmers and placed into a windows server/secure facility. From here the SAS programmer can develop the classification levels of the SDTM Domains (demographic, event, interventions and findings) with SAS BI.
- After this, the interlinking of domains can be achieved using either a method to build a stored process in enterprise guide which interlinks the different domains, or build an information map which interlinks the different domains by the SAS BI content developer.
- Now SAS Web Report Studio can be used by the SAS BI content developer and various reports can be produced for the clinical statistician/programmer.



